At the beginning of time, all the energy in the universe was contained in a single relatively small location. This intense concentration of energy represented a massive amount of what we call potential energy, where potential energy is stored energy due to its location or position, and it is now equal to the total amount of energy in the universe today. As time goes on, the energy has spread over the vast expanse of our universe.
As our universe continues to spread out, it contains less and less useful energy. As less useful energy is available, less work can get done. As water flows over a dam, it contains less useful energy as well. This decrease in useful energy over time is referred to as entropy, where entropy is the amount of unusable energy in a system, and a system is simply a collection of objects that make up a whole.
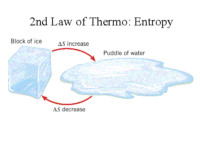
Entropy can also be referred to as the amount of randomness or chaos in a system – less organization. As usable energy decreases over time, disorganization and chaos increase. Thus, as stored potential energy is released, not all of it is converted into usable energy. All systems experience this increase in entropy over time. This is very important to understand, and this phenomenon is referred to as the second law of thermodynamics.
As you may have guessed, the second law of thermodynamics follows the first law of thermodynamics, which is commonly referred to as the law of energy conservation, and it states that energy can’t be created and it can’t be destroyed. In other words, the amount of energy in the universe, or any system, is constant. The second law of thermodynamics is commonly referred to as the law of entropy, and it holds that energy becomes less usable over time. Therefore, while the quantity, based on the first law, of energy remains the same, the quality of energy decreases over time, based on the second law.
Application Of The Second Law Of Thermodynamics
How does understanding the second law of thermodynamics help us? The implications of the second law of thermodynamics are as extensive as the boundaries of our universe. The universe is constantly losing usable energy and becoming more chaotic – less organized. This would suggest that the universe is not eternal but rather has an end, both in space and time.
At a much smaller level, the springs of an old clock must be wound in order for the clock to tick and to tock. The wound springs contain a lot of stored potential energy, and that energy is used over time to make the clock work – that’s what moves the hands of the clock. However, not all the potential energy is converted into usable energy. Some of that energy is lost in the form of heat – heat is unusable energy. If no one’s available to wind up the clock, it won’t be able to do any work again.
Let’s now consider a biological example. Our bodies are systems. As long as we can consume energy, our bodies can do the work that’s needed to survive and reproduce. Eventually, however, our bodies become less organized and more chaotic. Structure and function eventually give way to entropy. Nobody lives forever, and, just like the universe, our lives have an end.
However, scientists are constantly researching for ways to slow down entropy, both in man-made and natural systems. A good example of that is the internal combustion engine that makes our automobiles move. They’re far more efficient now than they were when they were first invented.
Lesson Summary
The first law of thermodynamics reminds us that the amount of energy in a system is constant. It has been the same since the beginning of time. Energy can be changed from one form to another, but it cannot be created or destroyed. That’s the first law.
The second law of thermodynamics tells us that entropy increases in a system as the potential energy is converted into usable energy for work. In other words, all systems, both natural and man-made, will eventually fall apart.

 Suggerimenti su come gestire il tempo durante la prova di matematica dell’Esame di Stato
Suggerimenti su come gestire il tempo durante la prova di matematica dell’Esame di Stato